Tocilizumab for in-patients
General Information
Drug class: Monoclonal antibody (Anti IL-6)
Restricted: For patient with COVID-19 infection who requiring oxygen see Patients in hospital with COVID-19 requiring oxygen
Tocilizumab can be initiated by all consultants in acute general medicine areas, infectious diseases, respiratory HDU and Critical Care.
A Blueteq form must be completed.
The first line tocilizumab product for the trust is RoActemra©. Prescribe RoActemra© on EPR and when completing the Blueteq form, select the Tocilizumab (RoActemra) option.
Refer to the Summary of Product Characteristics (SmPC) for tocilizumab for special warnings and precautions for use, although some may not be relevant for use in the acute setting, as the licensed indications address long-term use.
Dosage
Standard Dose
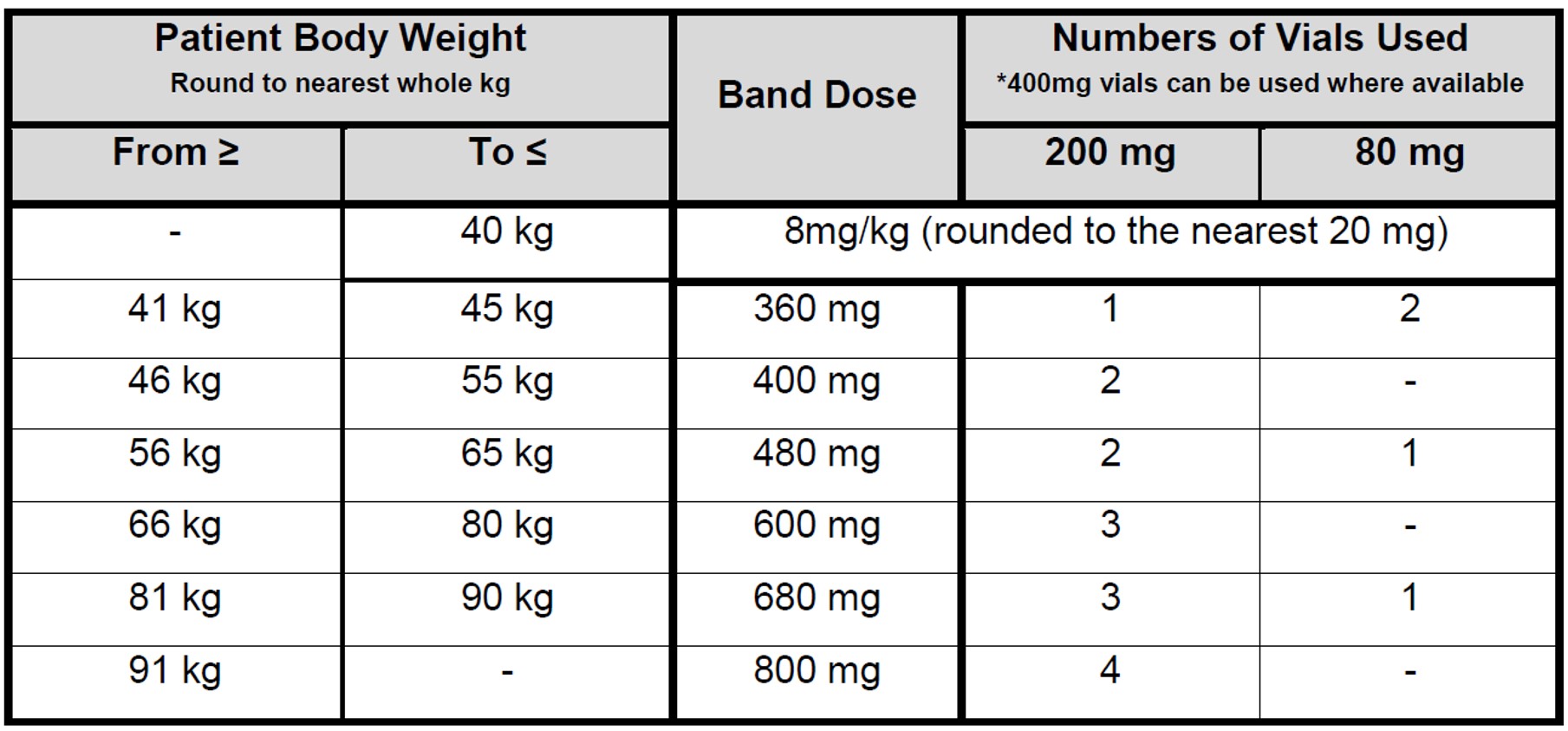
- The recommended dose is 8mg/kg STAT iv (use actual body weight). The maximum dose is 800mg.
Renal Impairment
- No dose adjustment necessary. Monitor renal function regularly post dose.
Dosing in obesity
- For individuals whose body weight is more than 100 kg, doses exceeding 800 mg per infusion are not recommended.
Use in hepatic impairment
- Caution should be exercised when considering tocilizumab treatment in patients with elevated ALT or AST more than 1.5 x Upper Limit of Normal (ULN).
- Not recommended for use if liver enzymes are 10 or more x ULN.
Notable Interactions
A useful resource is University of Liverpool COVID-19 Drug Interactions website
Avoid concurrent use with other immunosuppressive monoclonal antibody therapies.
Avoid concurrent use with live vaccines and live attenuated vaccines. Live and live attenuated vaccines should also be avoided for 1 year after use of tocilizumab. Discuss with Micro/ID.
This is not a complete list. Please see the BNF or speak to a pharmacist.
Additional information
- Women of childbearing potential must use effective contraception during and up to 3 months after treatment. Patients should be counselled regarding using effective contraception for up to 3 months after treatment. This must be documented on the Medicines Reconciliation form.
- Tocilizumab is not recommended in patients with COVID-19 where:
- Absolute Neutrophil Count less than 1x109/L
- Platelet Count less than 50x103/uL
- Note C-reactive protein (CRP) response may be suppressed for up to 3 months after administration of tocilizumab. Monitor for sign of infection.
- The name and batch number of the administered product should be recorded for traceability of biological medicinal products.
- Ensure that discharge letters to primary care, and other handovers between care settings, explicitly record the treatment that has been given, together with the dose and date of administration.
References
- RoActemra 20mg/ml Concentrate for Solution for Infusion [online]. Manufacturer – Roche Products Ltd. Last updated: 2022 Nov 22. Accessed via: www.emc.medicines.org.uk [Accessed 2023 Aug 04]
- National Institute for Health and Care Excellence. Technology appraisal guidance 878: Casirivimab plus imdevimab, nirmatrelvir plus ritonavir, sotrovimab and tocilizumab for treating COVID-19. Last updated: 2023 Jun 22. Accessed via: www.nice.org.uk. [Accessed 2023 Aug 04]
- Department of Health and Social Care. Independent report: Defining the highest risk clinical subgroups upon community infection with SARS-CoV-2 when considering the use of neutralising monoclonal antibodies (nMABs) and antiviral drugs. Last updated: 2023 Apr 05. Accessed via: www.gov.uk [Accessed 2023 Aug 08]
- Stockley’s Drug Interactions [online]. Accessed via: www.medicinescomplete.com. [Accessed 2023 Aug 04]
